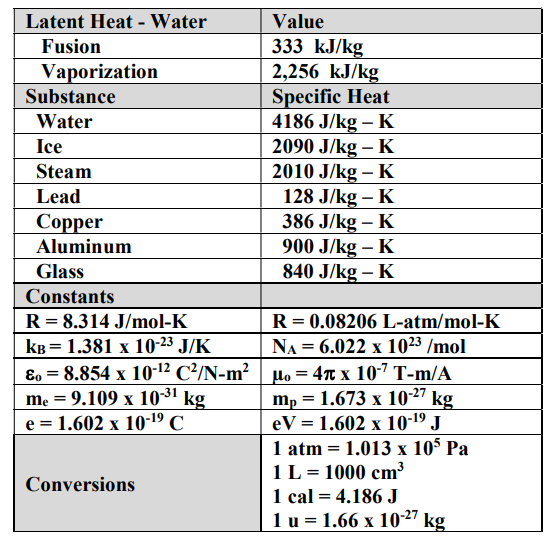
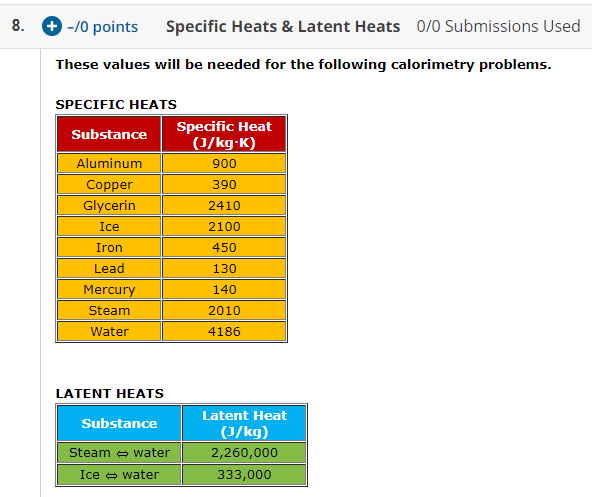
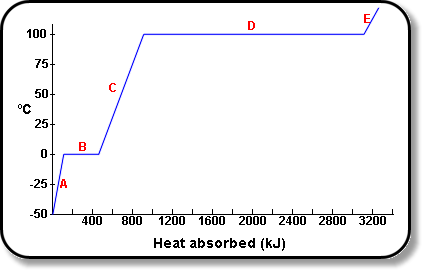
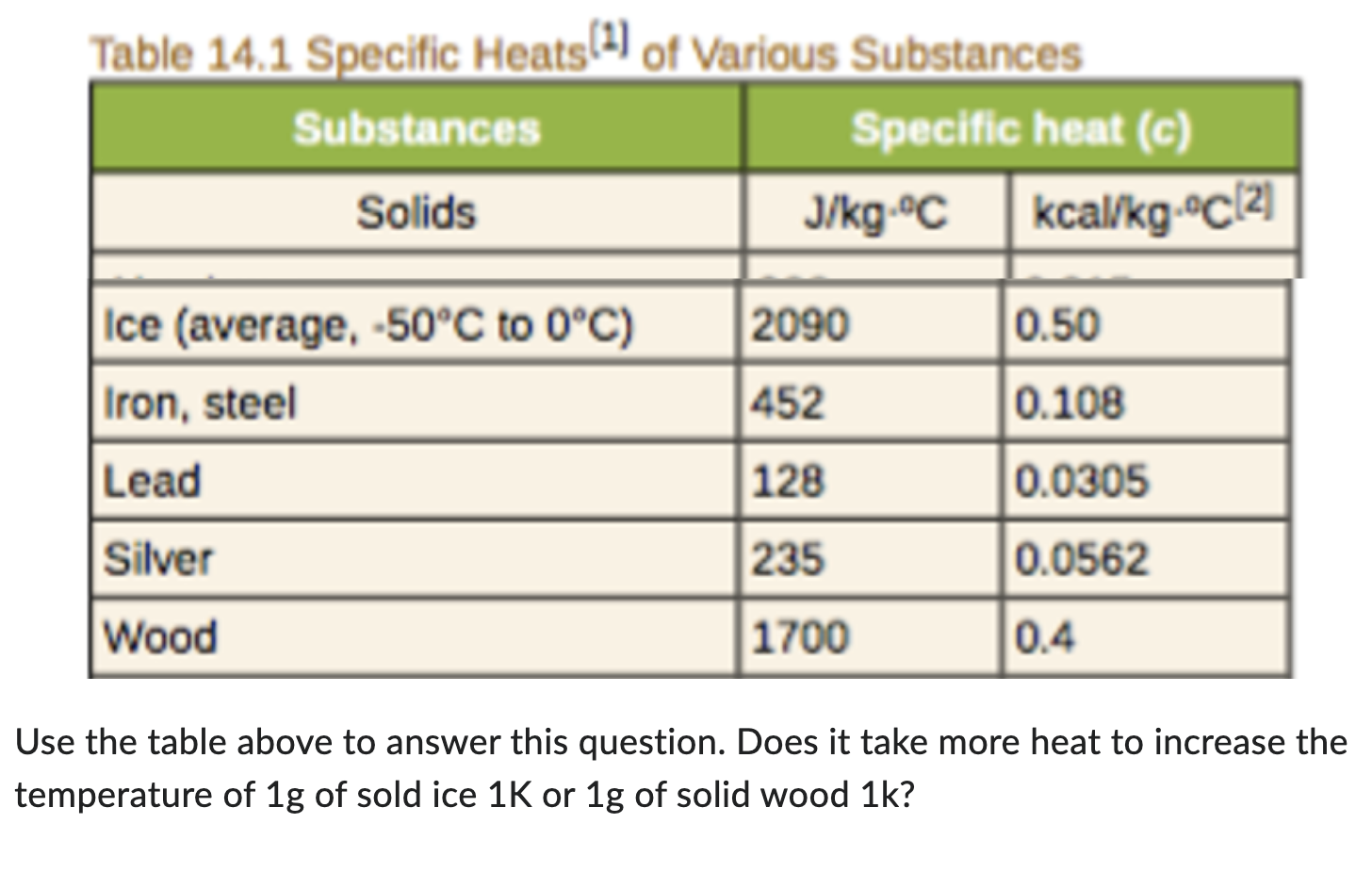
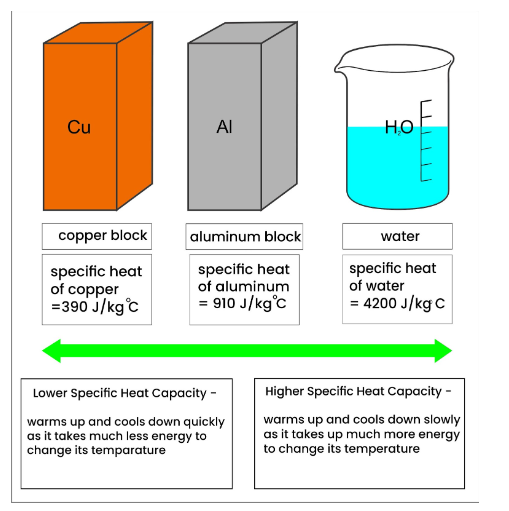
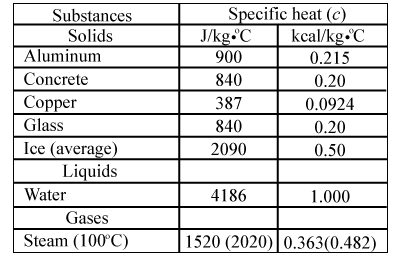
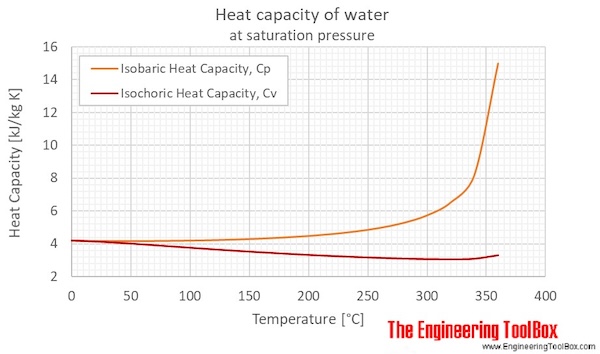
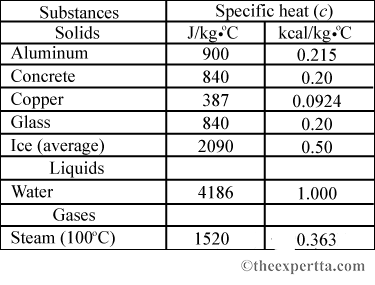
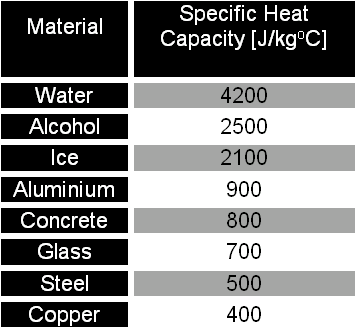
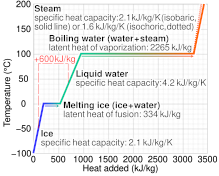
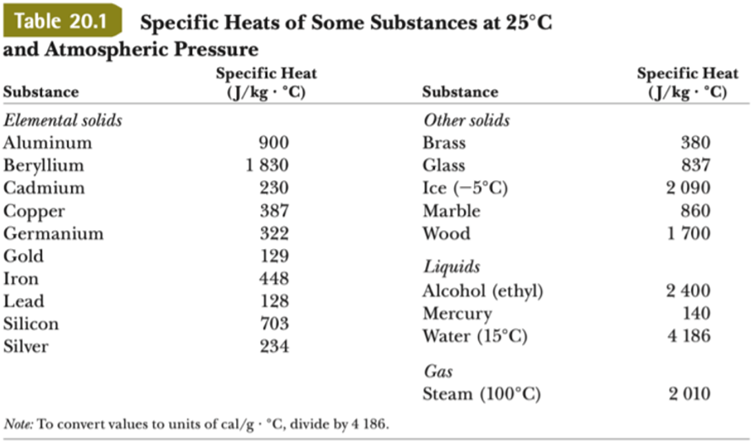
1 PHYS1001 Physics 1 REGULAR Module 2 Thermal Physics HEAT CAPACITY LATENT HEAT What is cooking all about? ptC_heat.ppt. - ppt download
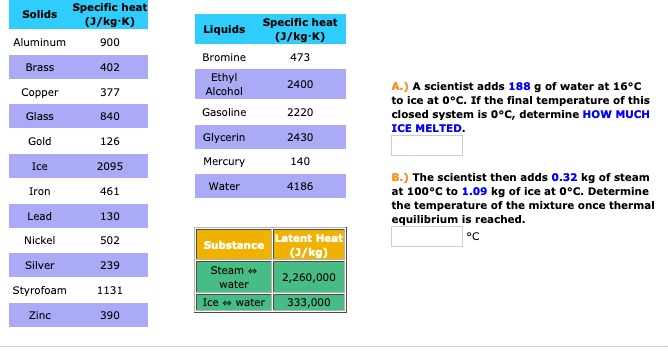
SOLVED: Specific heat Solids (J/kg K) Specific heat Liquids (J/kg K) Bromine 473 Ethyl Alcohol 2400 Aluminum 900 Brass 402 Copper 377 Glass 840 Gasoline 2220 Gold 126 Glycerin 2430 Mercury 140

A 40 g ice cube (latent heat of fusion = 3.33 x 10^5 J/kg) floats in 200 g of water (specific heat = 4184 J/kg degree Celsius) in a 100 g copper (
What is the difference between specific latent heat of melting of ice and specific latent heat of fusion of ice? - Quora
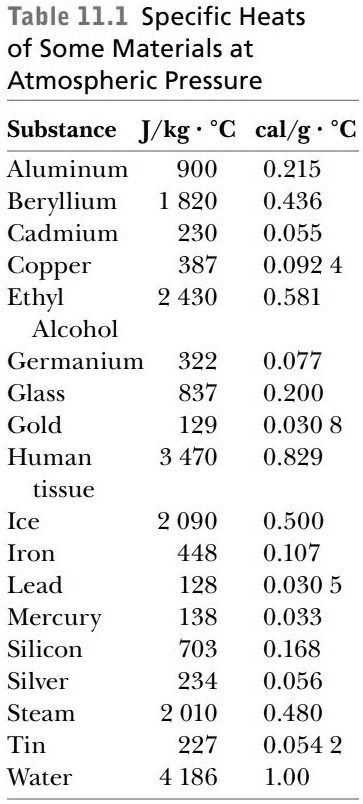
SOLVED: Table 11.1 Specific Heats of Some Materials at Atmospheric Pressure Substance J/kg 'C cal/g Aluminum 900 0.215 Beryllium 820 0.436 Cadmium 230 0.055 Copper 387 0.092 4 Ethyl 2 430 0.581

When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature. - ppt download